Properties of Carbon
Properties of Carbon
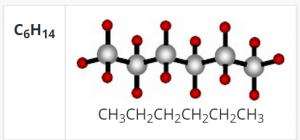
Without the element carbon, life as we know it would not exist. Carbon provides the framework for all tissues of plants and animals. These tissues are built of elements grouped around chains or rings made of carbon atoms.

Carbon is the fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. It is also the 15th most abundant element on earth and is present in all known life forms.
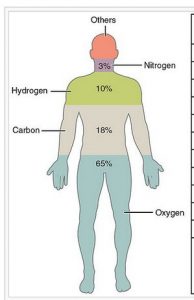
In the human body, carbon is the second most abundant element by mass (about 18.5%) after oxygen.
This abundance, together with the unique chemical bonding abilities of carbon make this element the chemical basis of all known life.
Carbon is the predominant atom or element found in all living systems. Why is this the case? As we noted above, Carbon has the unique property of being able to form 4 chemical bonds with other atoms (including Carbon). It is this ability that facilitates the formation of large ‘macro-‘molecules containing thousands of atoms.

As noted above, all biological molecules are based on the presence of carbon and hydrogen atoms. As we continue to explore biological molecules, it will be important to understand that there are essentially Four (4) main classes of biological molecules which we will explore in more detail. These classes are:
- Carbohydrates-Used for energy storage and transport
- Proteins- Used for structure, energy storage, and facilitating chemical reactions
- Lipids- Used for energy storage, formation of cell membranes and as hormones
- Nucleic Acids- Used to store and carry genetic information
Properties of Carbon
In this section, we will explore the unique properties of the Carbon atom which make it so important to support life on earth.
Section Summary
In this section we have learned the following:
- Carbon is an essential component of all living forms
- Carbon is one of the most abundant elements found in the Universe and comprises 18.5% (by mass) of the human body
- Carbon’s ability to form 4 chemical bonds with other atoms (including Carbon) is a key reason why it serves as a critical building block for large ‘macromolecules’, including:
- Carbohydrates
- Proteins
- Lipids
- and Nucleic Acids