Classification of Fats – Oils, Fats, and Waxes
Oils, Fats, Waxes
Oils, fats and waxes are used in biological systems primarily for energy storage (fats, oils) or for protection (waxes). These water-insoluble substances consist entirely of carbon, hydrogen and oxygen atoms.
Fats and Oils
One of the key building blocks of oils and fats are long chains of hydrocarbons called ‘fatty acids’ as shown below.
![]()
When three (3) fatty acid molecules are combined with a single 3-carbon glycerol molecule, this forms a larger fat or oil molecule referred to as a ‘triglyceride’ (see Figure 3-12 from your text) below:
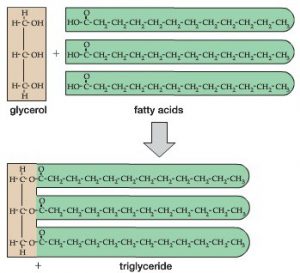
Both fats and oils serve primarily as energy storage molecules in biological systems due to their insolubility in water and tightly packed nature. In fact, fats and oils contain more than twice as many calories per gram (9 Cal/gram) as do proteins or carbohydrates (4 Cal/gram).
Differences between Fats and Oils
So what is the difference between a ‘fat’ and an ‘oil’ molecule? In the most simple sense, fats are solid at room temperature while oils are liquid. This is due to structural differences in the long-chain fatty acids of the triglyceride molecules.
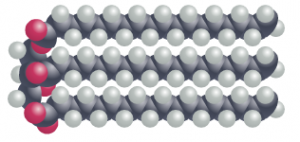
In fat molecules, all of the fatty acid chains are linear and straight. This allows the fat molecules to pack more tightly together, forming solid structures. In this configuration, each carbon atom in the fatty acid chains are bonded to two hydrogen atoms – a characteristic referred to as ‘saturated’. Saturated fats, found in food such as butter and lard, are the most unhealthy for us to consume.
In oil molecules, the fatty acid chains may contain one or more carbon-carbon double bonds; these double bonds introduce small ‘kinks’ into the chain causing the oil molecule to take up more physical space. Since oil molecules cannot be packed as tightly together as fat molecules, the consistency is more fluid.
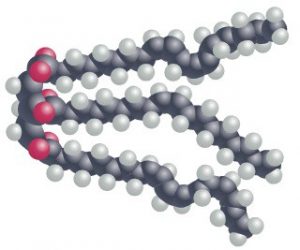
Thee carbon-carbon double bonds also reduce the number of hydrogen atoms found in the fatty acid – a characteristic referred to as ‘unsaturated’. A fat molecule which contains more than one carbon-carbon double bond is referred to as ‘polyunsaturated’.
Waxes
Waxes are chemically similar to highly-saturated fats and therefore are solid at room temperature. Waxes are typically used by plants to form waterproof coatings on leaves or stems. In animals, waxes are produced to provide waterproofing for fur or insect shells. Your likely familiar with the use of wax by honeybees to build honeycomb structures.

Lipids
- Lipids are water-insoluble molecules which can be categorized into several groups. These water-insoluble substances consist entirely of carbon, hydrogen and oxygen atoms.
- The Oils, fats and waxes are assembled from fatty acid chains combined with a 3-carbon glycerol molecule.
- Oils, fats and waxes are used in biological systems primarily for energy storage (fats, oils) or for protection (waxes).
- Fats and waxes are solid at room temperature while oils are liquid.
- Fats and waxes are ‘saturated ‘, while liquids are ‘unsaturated ‘.