Organic Vs Inorganic
Organic vs. Inorganic Molecules
The key difference between organic and inorganic molecules is the presence of carbon and hydrogen found in organic molecules; inorganic molecules typically lack both carbon and hydrogen together (though they may contain either carbon or hydrogen). In general, inorganic molecules are much simpler in nature than organic molecules since they often lack the versatile properties of the carbon atom.
Examples of inorganic molecules include:
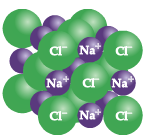 |
Na-Cl (Sodium Chloride; aka “Table Salt” |
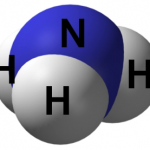 |
NH3 (Ammonia) |
 |
Na-F (Sodium Fluoride) |
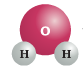 |
H2O (Water) |
In each of these examples, you will note the lack of Carbon (C) as a chemical component.
Examples of Organic Molecules
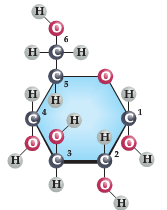
Glucose (shown at right) is an excellent example of an organic molecule. You will note the presence of 6 Carbon (C) atoms along with a total of 12 Hydrgogen (H) atoms and 6 Oxygen (O) atoms present in this ring-based structure (chemical formula = C6H12O6):
Glucose is used in biological systems as a key source of energy and is the base molecule used in all ‘carbohydrates’.
Properties of Organic Molecules
Most organic molecules consist of a number of Carbon, Hydrogen, Oxygen and Nitrogen atoms combined together to form larger molecular structures.
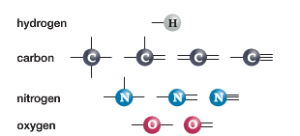
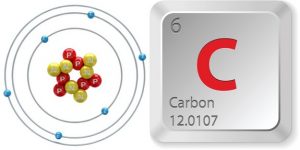
In the diagram, you will see how each of these atoms can form a number of chemical bonds (dark lines) with other atoms. In the case of Hydrogen, these atoms are capable of forming a chemical bond to one other atom. For Oxygen, they can form up to two chemical bonds. Nitrogen can form up to three chemical bonds while Carbon is capable of forming up to 4 chemical bonds. It is this unique property of Carbon (ability to form up to 4 bonds) which makes it such a flexible building block for all organic and biological molecules. Let’s examine the properties of Carbon in more detail now…
Organic vs. Inorganic Molecules
Please review the video below on the differences between organic and inorganic molecules
Section Summary
Organic molecules have a carbon backbone. They are so diverse because the carbon atom is able to form bonds with up to four other molecules.
