Diffusion
Introduction
To understand the movement of molecules across biological membranes, we first need to provide a few definitions.
- A solution contains both solutes and solvent
- A solvent is a liquid that is capable of dissolving solutes
- A solute is a compound that can be dissolved in a solvent
- Concentration refers to the amount of solute dissolved in a solvent
- A concentration gradient refers to differences between solute concentration on opposite sides of a membrane
Using these definitions, we can now establish two general rules to help us understand how materials move into or out of cells:
- Rule #1 – Solutes move “down” concentration gradients without the expenditure of energy. This means solutes tend to move from areas of high concentration to areas of low concentration. Since this movement doesn’t require energy, it is referred to as “passive” transport.
- Rule #2 – Solutes can move against their concentration gradients (from areas of low concentration toward areas of high concentration), but this requires the input and expenditure of energy. This movement is called “active” transport.
Organisms rely heavily on both passive and active transport to import and export nutrients, gases, and wastes. Active transport, which requires energy, plays an essential role in allowing organisms to import raw materials, such as glucose, from the environment where they exist in lower concentrations than what is found inside the cell. Without active transport, organisms could not survive.
Let’s now see how these rules dictate the principle of ‘diffusion’…
Diffusion
Now that we have explored the rules of molecular movement, we can now explore the principle of diffusion.
Consider placing a drop of dye into a beaker filled with water. In this instance, the droplet of dye represents a solute being placed into a solvent (the water). In a short period of time, the dye molecules will, on their own, disperse throughout the solvent eventually establishing a uniform concentration throughout the beaker. The process of dispersing solutes to achieve uniform concentration is called ‘diffusion’.
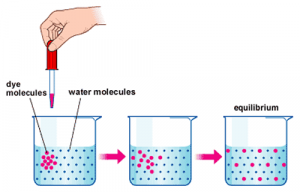
The fundamental principles of diffusion are:
- Diffusion describes the net movement of molecules from an area of high concentration toward areas of lower concentration
- The greater the concentration gradient, the faster the rate of diffusion that will occur
- Higher temperatures cause molecules to move faster and consequently result in faster rates of diffusion
- Diffusion will continue until a uniform concentration is achieved
Summary
Diffusion describes the net movement of molecules from an area of high concentration toward areas of lower concentration. The greater the concentration gradient, the faster the rate of diffusion that will occur.
Factors such as higher temperatures cause molecules to move faster and consequently result in faster rates of diffusion. Diffusion will continue until a uniform concentration is achieved.
Diffusion
The video below discusses the process of diffusion and how factors such as temperature and concentration affect the rate of diffusion in a solution.