Carbohydrates
Introduction
Carbohydrates are the major source of food and the major form of energy for most living organisms. When individual carbohydrate molecules combine together to form larger structures (or polymers) they can also serve as long term food storage (i.e. starch), as protective coverings (i.e. chitin) and as the main structural support for plants (i.e. cellulose).
Monosaccharides and Disaccharides
The term ‘carbohydrate‘ literally means ‘carbon plus water’. Therefore, carbohydrate molecules are composed entirely of carbon, hydrogen and oxygen atoms, typically in a ratio of (1) carbon atom: (2) hydrogen atoms: (1) oxygen atom. The carbohydrate glucose has the chemical formula C6H12O6 and therefore is consistent with this ratio. In most instances, carbohydrates exist in the form of small, water-soluble sugars which can be combined together to form larger molecules or longer chains of molecules.
The number of carbon atoms found in a monosaccharide (mono- means one) range from 2 to 7 and the names of the monosaccharides usually end with the suffix –ose. Glucose is the most important sugar utilized by humans. The energy released when glucose is broken down in the human body is utilized to make the energy molecule ATP. Plants have the ability to make glucose by the process of photosynthesis. The chlorophyll (green pigment) in plants captures the light energy from the sun and utilizes that to combine carbon dioxide (CO2) and water (H2O) into glucose (C6H12O6).
Disaccharides (di- means two) form when two monosaccharides bond together. The most common disaccharide used by humans is the molecule sucrose. This consists of two glucose molecules bonded together by a glycosidic bond.
A disaccharide is formed by a dehydration reaction. This means that by combining the two molecules together, water is formed (de-removing, hydration-water). A hydroxyl group (-OH) from one molecule joins with a hydrogen (H-) from the other molecule (making water) and results in a covalent bond joining the two glucose molecules.
Glucose and Sucrose
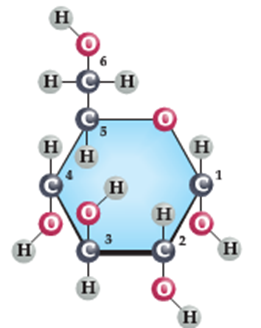
The figure above shows glucose the monosaccharide on the left and on the right, two glucose molecules joined together to form the disaccharide, sucrose.
Polysaccharides
Polysaccharides, as their name implies, are complex sugars comprised of many monosaccharide molecules linked together. These larger molecules are typically insoluble in water at room/body temperature and provide cells with the ability to store energy, as is the case with the molecule starch. Another key function of polysaccharides is structural. For example, the ‘crunch’ of celery is due to the presence of cellulose, a large polysaccharide found only in plants, within the outer walls of the plant’s cells.
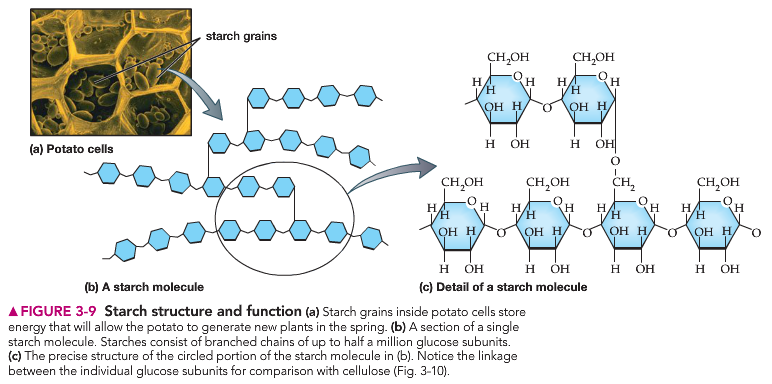
You can see how the individual monosaccharide molecules are linked together to form branched (starch) and unbranched (cellulose) polysaccharides.
Cellulose
Cellulose is the main component of cell walls. It is made of long chains of glucose that are held together by cross linkages. These are bonds that link the long glucose chains together.
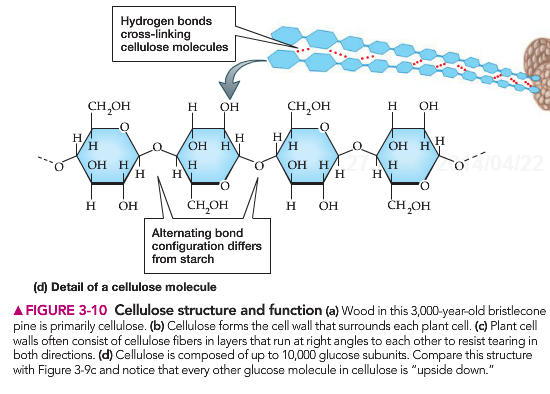
Due to the structural position of these glucose molecules, humans cannot digest cellulose. This means that it “leaves” the human body as the same structure as it entered. This forms the FIBER in our diet. It allows the digestive track to grab on to material and push it out. Humans do not have the digestive enzymes necessary to break down the cellulose. Certain animals possess this enzyme and along with the digestive bacteria present in their body can break down this plant material and utilize cellulose as an important ingredient in their diets.
Summary
- Carbohydrates are a family of biomolecules that include sugars, starches, cellulose, and chitin.
- Sugars include monosaccharides (such as glucose) and disaccharides (such as sucrose). They are used for temporary energy storage and the construction of larger biomolecules, including complex carbohydrates.
- Starches (found in plants) and glycogen (found in animals) are polysaccharides which provide long-term energy storage.
- Cellulose forms the cell walls of plants and provides rigidity and structure. Similarly, chitin strengthens the exoskeletons of many invertebrates.
Sources:
Figures from OpenStax Biology 2e, Volume 1. Licensed under: CC-BY:Attribution