Lipids
Introduction

Lipids represent a large group of molecules which consist almost entirely of carbon and hydrogen atoms. They include fats, oils, phospholipids, and steroids. A key distinguishing feature of lipids is the fact that they are insoluble in water – a characteristic termed ‘hydrophobic’ which means “water fearing” (hydro = water; phobic = fearful).
Lipids have a number of functions in cells. They provide long term storage for energy and they serve as an insulation layer for water mammals. Their hydrophobic nature help keep plants and animals dry in wet environments. Many hormones are composed of lipids and lipids are an important component of cellular membranes.
The key functions of lipids in biological systems include:
- Energy Storage
- Water Barrier/Protection
- Cell Membranes
- Hormones
Learning
Lipids can be categorized into (3) functional groups:
Oils, Fats, Waxes
Energy storage, protection
Phospholipids
Construction of cell membranes
Steroids
Hormones
Oils, Fats, Waxes
One of the key building blocks of oils and fats are long chains of hydrocarbons called ‘fatty acids’ as shown at right.
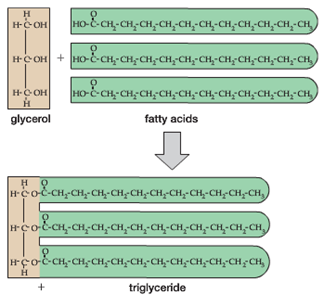
When three (3) fatty acid molecules are combined with a single 3-carbon glycerol molecule, this forms a larger fat or oil molecule referred to as a ‘triglyceride’
Both fats and oils serve primarily as energy storage molecules in biological systems due to their insolubility in water and tightly packed nature. In fact, fats and oils contain more than twice as many calories per gram (9 Cal/gram) as do proteins or carbohydrates (4 Cal/gram).
Differences Between Fats and Oils
So what is the difference between a ‘fat’ and an ‘oil’ molecule? In the most simple sense, fats are solid at room temperature while oils are liquid. This is due to structural differences in the long-chain fatty acids of the triglyceride molecules.
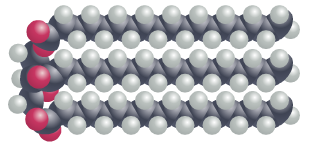
In fat molecules, all of the fatty acid chains are linear and straight. This allows the fat molecules to pack more tightly together, forming solid structures. In this configuration, each carbon atom in the fatty acid chains are bonded to two hydrogen atoms – a characteristic referred to as ‘saturated’. Saturated fats, found in food such as butter and lard, are the unhealthiest for us to consume.
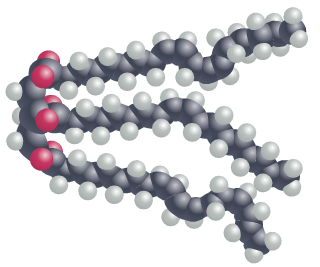
In oil molecules, the fatty acid chains may contain one or more carbon-carbon double bonds; these double bonds introduce small ‘kinks’ into the chain causing the oil molecule to take up more physical space. Since oil molecules cannot be packed as tightly together as fat molecules, the consistency is more fluid.
Three carbon-carbon double bonds also reduce the number of hydrogen atoms found in the fatty acid – a characteristic referred to as ‘unsaturated’. A fat molecule which contains more than one carbon-carbon double bond is referred to as ‘polyunsaturated’.
Fats and oils are forms of lipids. Lipids include triglycerides, phospholipids, and sterols. Fats have several important roles in the body. They provide energy and essential fatty acids for hormone production and nervous system health, transport fat-soluble vitamins, regulate cell function, maintain cell membrane integrity, protect vital organs, and contribute to flavor and satiety of meals.
Triglycerides are the most common dietary fat and are composed of glycerol and three fatty acids. The fatty acids can be classified on the basis of chain length, level of saturation, and shape. Saturated fatty acids have no double bonds and are solid at room temperature. These are found predominately in animal-based foods. Unsaturated fatty acids contain one or more double bonds and are liquid at room temperature. These are found predominately in plant-based foods. A cis fatty acid has hydrogen atoms on the same side of the double bond, while a trans fatty acid has hydrogen located on opposite sides of the double bond. Trans fats, made from unsaturated oils through a process called hydrogenation, are solid at room temperature. These are used by the food industry to enhance flavor, texture, and shelf life
Waxes
Waxes are chemically similar to highly-saturated fats and therefore are solid at room temperature. Waxes are typically used by plants to form waterproof coatings on leaves or stems. In animals, waxes are produced to provide waterproofing for fur or insect shells. Your likely familiar with the use of wax by honeybees to build honeycomb structures.
Phospholipids
Structurally, phospholipids are similar, yet distinct, from oils and fats.
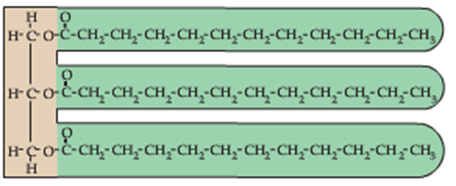
Recall that the structure of a fat/triglyceride is based on three (3) fatty acid chains attached to a (3)-carbon glycerol backbone as shown below:
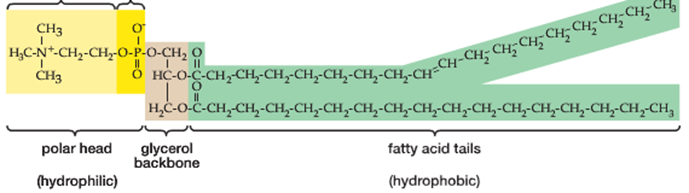
A phospholipid, in contrast, is constructed from (2) fatty acid chains attached to a (3) carbon glycerol backbone as shown:
Instead of finding a 3rd fatty acid chain attached to the first carbon in the glycerol backbone (top left in the picture above), phospholipids contain a single ‘phosphate functional group’. This functional group contains a single phosphorous atom (P):
Behavior of Phospholipids
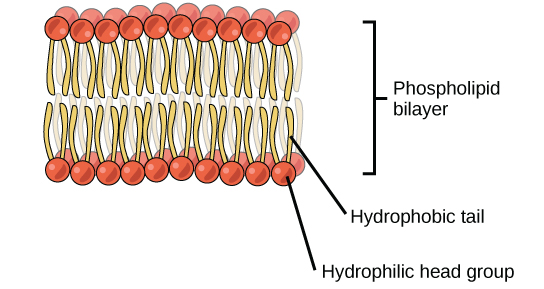
A key distinguishing feature of a phospholipid, therefore, is that it has both a hydrophilic’ and a ‘hydrophobic’ (water-fearing) end. When phospholipids are mixed in water, they will naturally orient themselves so that the hydrophilic ends (the ‘head’) will point outward, facing the water while the hydrophobic ends (the ‘tails’) will tuck themselves inward forming a structure called a ‘micelle’. This automatic orientation is an important property used to form cell membranes which will be discussed later in the class.
Steroids
Steroids are special types of lipids which are used by the body as hormones. There are several different types of steroids including:
- Cholesterol
- Testosterone
- Estrogen
Steroids have a fused ring structure and are grouped with fats due to the fact that they are hydrophobic. They have four linked carbon rings and usually a short tail.
Cholesterol is the most common steroid and is made in the liver. It is important as it is a precursor to many hormones such as testosterone and estradiol. Cholesterol in animals and phytosterol in plants are important components in their cellular membranes.
Summary
- Lipids are water-insoluble molecules which can be categorized into several groups. These water-insoluble substances consist entirely of carbon, hydrogen and oxygen atoms.
- The Oils, fats and waxes are assembled from fatty acid chains combined with a 3-carbon glycerol molecule.
- Oils, fats and waxes are used in biological systems primarily for energy storage (fats, oils) or for protection (waxes).
- Fats and waxes are solid at room temperature while oils are liquid.
- Fats and waxes are ‘saturated ‘, while liquids are ‘unsaturated ‘.
- Phospholipid molecules have two distinct ends. A hydrophobic (water-hating) end and a hydrophilic (water-loving) end. This makes it a perfect molecule in forming a cell membrane.
- Cholesterol is the most common steroid found in the human body.
Sources:
“Lipids.” By OpenStax Biology 2e. Retrieved from: https://openstax.org/books/biology-2e/pages/3-3-lipids/ Licensed under: CC-By: Attribution
“Biological Molecules.” By OpenStax Concepts in Biology. Retrieved from: https://openstax.org/books/concepts-biology/pages/2-3-biological-molecules Licensed under: CC-By: Attribution