Proteins
Introduction
Proteins are the most diverse class of biological molecule. They are composed of long chains of smaller subunits called ‘amino acids’ and proteins can be ‘folded’ into many different structural configurations, resulting in a vast array of possible functions provided to the cell.
Let’s take a closer look now…
Learning
Types and Functions of Proteins
Proteins are the most abundant biological macromolecule in all living things and have a variety of functions. Three types of proteins are explained here:
Enzymes
Enzymes are proteins that lower the amount of energy needed in a chemical reaction. Enzymes are specific for the substrate that it works with. They also bring the reactants together. They help in the breakdown, rearrangement, or synthesis of molecules. An example of an enzyme is amylase which helps break down starch into smaller usable units.
Hormones
Hormones are biochemical molecules that are made in one part of the body in endocrine glands and then travel to target organs. They control many physiological processes like growth, metabolism, and reproduction. Insulin is a hormone made in the pancreases that helps cells control blood glucose levels.
Amino Acids
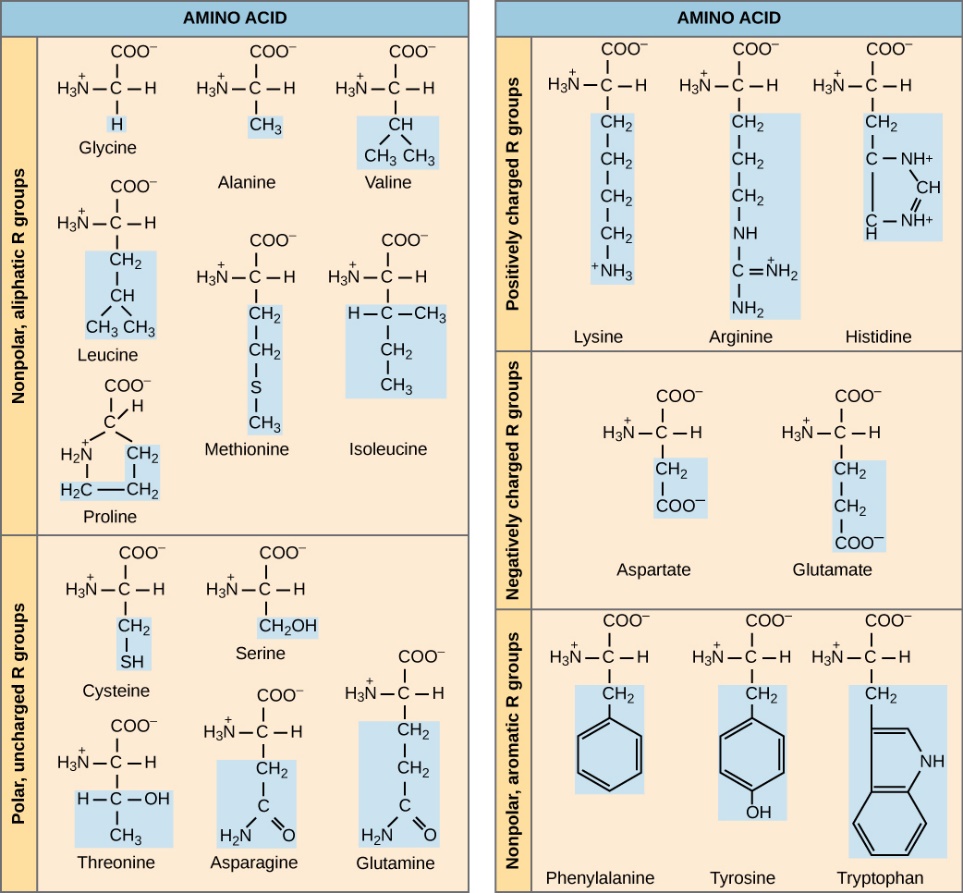
Amino acids are the units that make up proteins. There are twenty amino acids that that can be bonded together to make a protein. They have a basic structure of carbon in the center, an amino group on one side of the carbon, and a carboxyl group on the other side and different side chains off the central carbon. These side chains are what makes each amino acid different. Out of the twenty different amino acids, nine of them are considered essential. They are called that since humans cannot produce those, therefore they must be ingested.
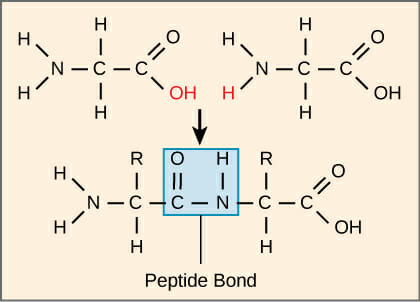
The sequence and number of amino acids found in a protein determines its shape, and its function. When the amino acids join a covalent bond, called a peptide bond is formed. The -OH group from one amino acid and the -H from the other amino acid are removed allowing the two amino acids to form the peptide bond.
This is called dehydration synthesis as a the -OH and -H join to form a water molecule (H2O).
A combination of the twenty amino acids joining together in a chain. (by dehydration synthesis). The resulting molecule is a polypeptide. This peptide is then modified in various ways. It will be modified into a distinct shape and for a unique function to become a protein.
Summary
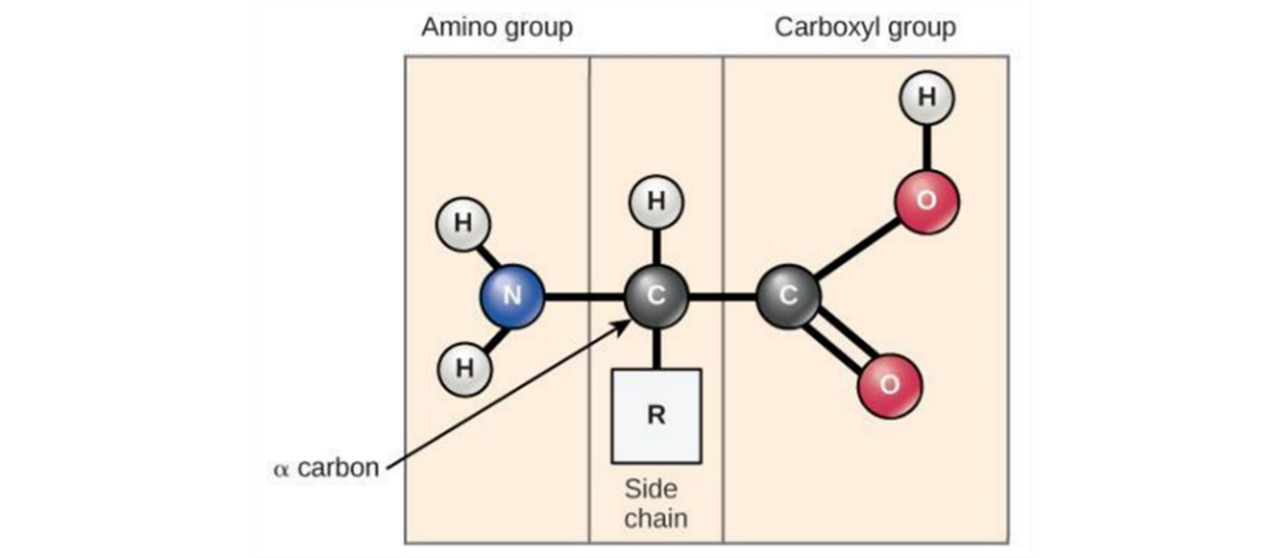
Proteins are composed of a series of smaller subunits called ‘amino acids’ which are joined together into a long chain. If we examine the chemical structure of an amino acid, we can see that it is based upon a central carbon atom bonded to three (3) discrete groups along with a hydrogen atom:
- On the left side of the amino acid, we find an ‘amino group’ which is based upon the nitrogen atom.
- On the right side of the amino acid, we find a ‘carboxylic acid group’.
- At the top of the amino acid, we find the presence of a ‘variable group’. There are (20) different groups which can be found in this position on the amino acid generating a total of (20) different amino acids in nature. Some of these groups can be hydrophobic, some hydrophilic while others can form strong chemical bonds with one another.
Sources:
“Biological Molecules.” By Concepts of Biology. Retrieved from: https://openstax.org/books/concepts-biology/pages/2-3-biological-molecules Licensed under: CC-BY: Attribution (applicable license here)
“Proteins.” By OpenStax Biology 2e. Retrieved from: https://openstax.org/books/biology-2e/pages/3-4-proteins/ Licensed under: CC-BY: Attribution
